
For pharma sector
We study vaccines with decades of experience and internationally recognized, high-quality scientific expertise. Our comprehensive network of clinics offers first-class prerequisites for this. FVR offers clinical vaccine research expertise aimed at obtaining a license for marketing authorization (phases 1–3), as well as post-licensure investigations into the effectiveness and safety of vaccines already in use (phase 4). We have strong expertise in Real-World Evidence (RWE) studies and pragmatic vaccine trials, utilizing Finland’s comprehensive health registers and data resources.
Why work with FVR – Finnish Vaccine Research?

phases 1-4.
- Capability to enroll high volumes of participants for clinical trials (thousands) and large pragmatic trials (tens of thousands)
- Decades of vaccine study experience from >160 studies throughout all age groups
- Strong references for post-licensure studies, favourable Finnish operating-environment
- Core focus on vaccine trials
- Continuous development mindset, agility and drive to innovate together with partners
- Quick start-up processes and efficient administration

across Finland, with decades of accumulated experience
| No. of new launched studies each year, working with major vaccine manufacturers/big pharma R&D | 10-15 |
| Total no. of ongoing studies/ year | 26 (in 2023), 11 different pathogens |
| Average no. of new enrolled volunteers /year (top year) | Top year for clinical trials 11,000, for pragmatic trials >30,000 |
Why study vaccines in Finland?

(enumeration) for linking individual-level health data


The reach of our clinics is not limited to their location as we also reach neighboring municipalities
| City/town | Population size, end-2023 |
| Espoo | 314,024 |
| Helsinki (2 clinics) | 674,500 |
| Järvenpää | 46,490 |
| Kokkola | 48,295 |
| Oulu | 214,633 |
| Pori (closure planned) | 83,106 |
| Seinäjoki | 66,160 |
| Tampere | 255,050 |
| Turku | 201,863 |
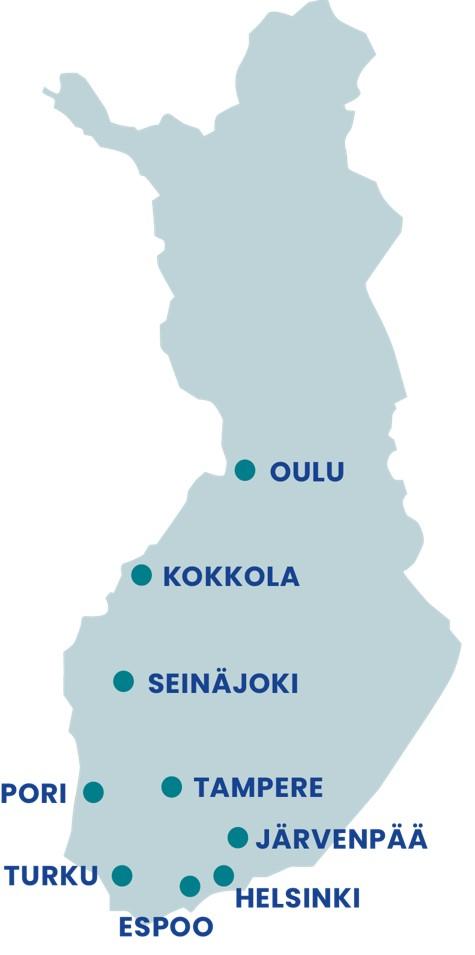
(Pori clinic closure planned)
Contact us
Arto Palmu
Chief Research & Medical Officer
Licentiate of Medicine, MD
Doctor of Philosophy, Ph. D.
Specialist in Public Health Medicine
University lecturer in clinical epidemiology
Publications:
96 original scientific articles, 3 supervised dissertations, opponent in two doctoral dissertations
https://orcid.org/0000-0001-8071-8896
Contact:
+358 50 5497 1113
arto.palmu (a) fvr.fi